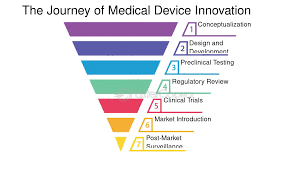
Bringing a new therapy or medical technology from concept to commercialisation is one of the most challenging and rewarding journeys in the life sciences industry. Each step, from early discovery to patient access, demands precision, collaboration, and compliance. For businesses and research teams, understanding the dynamics between innovation, funding, and regulation is critical to success.
Modern biopharma development is no longer confined to the lab bench. It’s an intricate ecosystem of data-driven insights, strategic partnerships, and evolving digital tools. With global health demands shifting rapidly, companies are increasingly adopting adaptive research models to accelerate progress while maintaining safety and quality standards.
The evolving landscape of medical innovation
The pharmaceutical industry is transforming at a pace never seen before. Artificial intelligence, automation, and cloud-based data integration are enabling scientists to predict efficacy and side effects earlier in the pipeline. This has significantly reduced time and costs associated with drug discovery, allowing smaller biotech firms to compete with industry giants.
At the same time, regulatory bodies are adapting their frameworks to accommodate emerging technologies. The rise of decentralised clinical trials and remote monitoring solutions is redefining how researchers gather and analyse data. These advances are also giving patients greater access to innovative treatments, particularly in underrepresented populations.
Strategic partnerships as a growth catalyst
Collaboration is now a cornerstone of progress. Biotech startups often rely on partnerships with larger pharmaceutical firms, academic institutions, and contract research organisations to navigate the complex development process. Strategic alliances not only bring financial support but also provide access to global infrastructure and regulatory expertise.
In a competitive and highly regulated market, partnerships can make or break a project. The ability to share data securely, align research goals, and maintain compliance across jurisdictions is vital. Companies that integrate digital collaboration platforms into their workflows are seeing measurable improvements in efficiency and transparency.
Balancing innovation with compliance
Innovation is meaningless without accountability. Every new therapy must adhere to strict regulatory frameworks to ensure patient safety and product efficacy. Global harmonisation initiatives, such as those led by the International Council for Harmonisation (ICH), are helping to streamline processes, but regional variations still present challenges.
Compliance demands continuous monitoring, detailed documentation, and a robust understanding of changing policies. This is where digital transformation plays a pivotal role — automating record-keeping, enabling predictive analysis for regulatory submissions, and reducing the administrative burden that often slows progress.
The bridge between science and success
In drug development, the transition between discovery and commercialisation hinges on one crucial stage — the clinical development phase. This stage determines whether a concept is safe and effective for human use, combining rigorous testing with ethical oversight. Businesses investing in innovation must understand that this is not just a scientific process but also a strategic one. It influences funding decisions, market potential, and long-term viability. As technology evolves, so does the efficiency and precision of this vital step toward medical advancement.
The future of therapeutic development
Looking ahead, the integration of machine learning and real-world evidence into drug development pipelines will revolutionise how therapies reach patients. Predictive modelling will guide decision-making, while precision medicine will tailor treatments to individual genetic profiles.
Moreover, sustainability is becoming a growing focus. The pharmaceutical industry is exploring greener manufacturing methods and reducing waste in research and production processes. Ethical considerations are also gaining prominence, ensuring that innovations serve humanity equitably.
The journey from laboratory discovery to market-ready treatment is complex, but with the right combination of technology, collaboration, and strategy, the possibilities are limitless. Companies that embrace agility and foresight will be best positioned to shape the future of global health.